Positive Control Synthesis
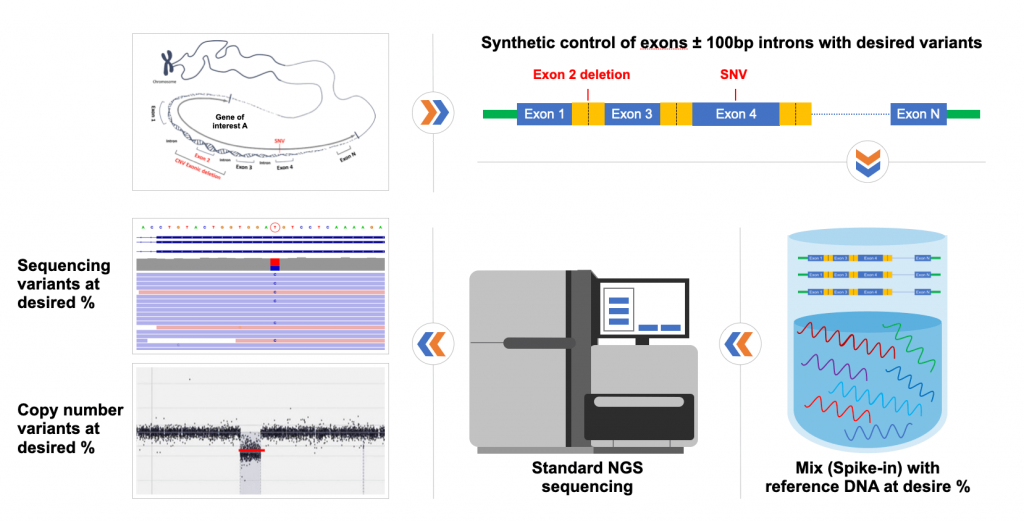
Figure legend:
This figure illustrates how the coding regions of your gene of interest are synthesized, and variants of interest are added at the same time. The variants can be SNV (substitution, deletion, duplication etc.) or CNV (deletion or duplication of single or multiple exons). The synthetic reference is used as spike-in controls, mixed with well-characterized human genomic DNA. Synthetic positive controls can also be full genes (WGS testing), exons plus specific intronic regions or human mitochondrial genome.
Why will you need our synthetic positive control product?
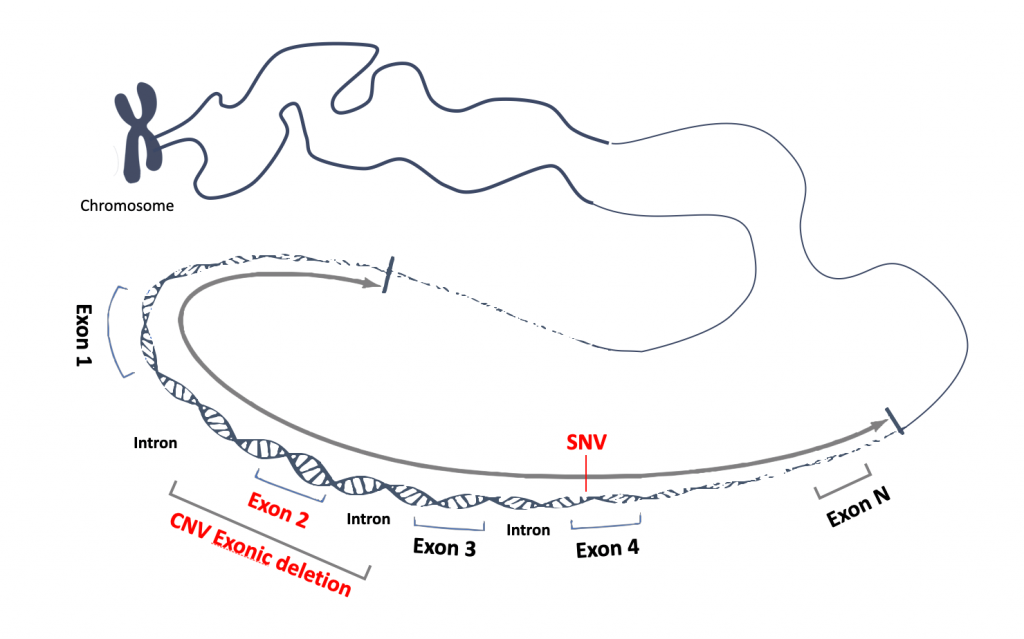
How our synthetic control works?
Coding regions of your genes of interest or whole genes can be synthesized, and your variants of interest will be added at the same time, The variants can be sequencing variants (SNV, including substitution, deletion, duplication etc.) or CNV, including deletion or duplication of single or multiple exons. Our products will provide consistent and reliable performance for NGS assay development, validation, and routine run QC in all the genetic laboratories and will be especially beneficial for CLIA/CAP regulated clinical laboratories.
Our synthetic reference materials can be used by itself and will comply with regular PCR or capture based NGS strategies. Or it can be used as spike-in controls, mixed with well-characterized human genomic DNA as background wild-type material. The later will be extremely useful if you are working on the validation study to establish the analytical sensitivity (lower detection limit) of your somatic assays.
Why choose Watsonbio?
What services are provided now?
- Service 1: Synthetic positive controls of your genes of interests using exonic regions plus 100 bp exon-intron boundary sequences and the desired variants will be added during the synthesis procedure. Since most genetics testing nowadays still focus on exonic regions, this will be most practical strategy.
- Service 2: Synthetic positive controls of full genes, this is mainly for WGS testing, and special tests that requires the intronic regions. Due to the large size and genetic complexity of intronic regions, some regions may have technical difficulties during synthesis.
- Services 3: Synthetic positive controls of human mitochondrial genome. Syntheses will be based on Revised Cambridge Reference Sequence (rCRS) of the Human Mitochondrial DNA. The desired sequencing variants and common recurrent deletions will be added the same time. The build-in amplification primers are the same as the universal long range PCR primers being used in most clinical laboratories.
